Introduction: This study delves into the therapeutic potential of the Enhancer of zeste homolog 2 (EZH2) degrader MS1943 and the Bruton's tyrosine kinase (BTK) inhibitor Ibrutinib in B-cell lymphomas. The study's primary objective was to compare the efficacy of MS1943, a Proteolysis-targeting chimera (PROTAC)-based degrader, with the FDA-approved EZH2 inhibitor Tazemetostat. The study also aimed to explore the potential synergistic effects of combining these therapies with Ibrutinib, a widely used BTK inhibitor in lymphoma treatment.
Materials and Methods: The study employed a range of lymphoma cell lines, including B-cell and T-cell lymphomas, which were treated with MS1943, Tazemetostat, and Ibrutinib, either alone or in combination. The impact of these treatments on cell viability was evaluated using the Cell Counting Kit-8 (CCK-8) assay at different time points and concentrations. Cell cycle distribution was assessed using flow cytometric analysis, and the expression of downstream effectors of the unfolded protein response (UPR) pathway, including Xbp1, Chop, and Bip, was examined using quantitative polymerase chain reaction (qPCR). Western blot analysis was performed to investigate the association with specific apoptosis-related pathways.
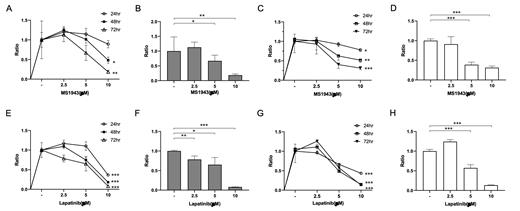
Results: The results demonstrated that MS1943 exhibited significantly greater inhibitory effects than Tazemetostat in all lymphoma cell lines tested, particularly in B-cell lymphomas. MS1943 treatment resulted in increased expression of UPR pathway effectors, suggesting a correlation between the observed anti-proliferative effects and the differential expression of these factors. The combination of MS1943 and Ibrutinib demonstrated significant inhibitory effects across all B-cell lymphoma cell lines, with the most prominent cytotoxicity observed after 72 hours of culture. The combination treatment resulted in an increase in the number of cells in the G2/M phase, accompanied by a decrease in the number of cells in the G0/G1 phase. Furthermore, the combination treatment led to a notably higher proportion of apoptotic and necroptotic cells compared to either individual treatment or the combination of Ibrutinib and Tazemetostat. The combination treatment resulted in an increase in the expression of p53 and PUMA, and a significant increase in miR29b expression.
Discussion: Our findings suggest that the simultaneous treatment of MS1943 and Ibrutinib in B-cell lymphoma induces apoptosis through the miR29b-mediated p53 upregulated pathway, thereby exhibiting potent anti-tumor effects. The study provides compelling evidence that MS1943 exhibits a pronounced inhibitory effect on B-cell lymphomas compared to Tazemetostat, highlighting its potential as a promising therapeutic agent in the treatment of B-cell lymphomas. The combination of MS1943 and Ibrutinib enhances apoptotic and necroptotic cell death, resulting in a significant reduction in viable cells. This study provides valuable insights into the mechanisms underlying the anti-tumor effects of this drug combination, highlighting the potential of MS1943 as an effective treatment option for lymphoma.
Disclosures
Jeon:Catholic University of Korea (10-2018-0112511, 10-1718380): Patents & Royalties; Janssen, F. Hoffmann-La Roche Ltd, Antengene, Celltrion, Vigencell: Consultancy; Korean research fundings: Research Funding; Korean Society of Experimental Hematology: Membership on an entity's Board of Directors or advisory committees; Catholic University of Korea, Yeouido ST. Mary hospital: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal